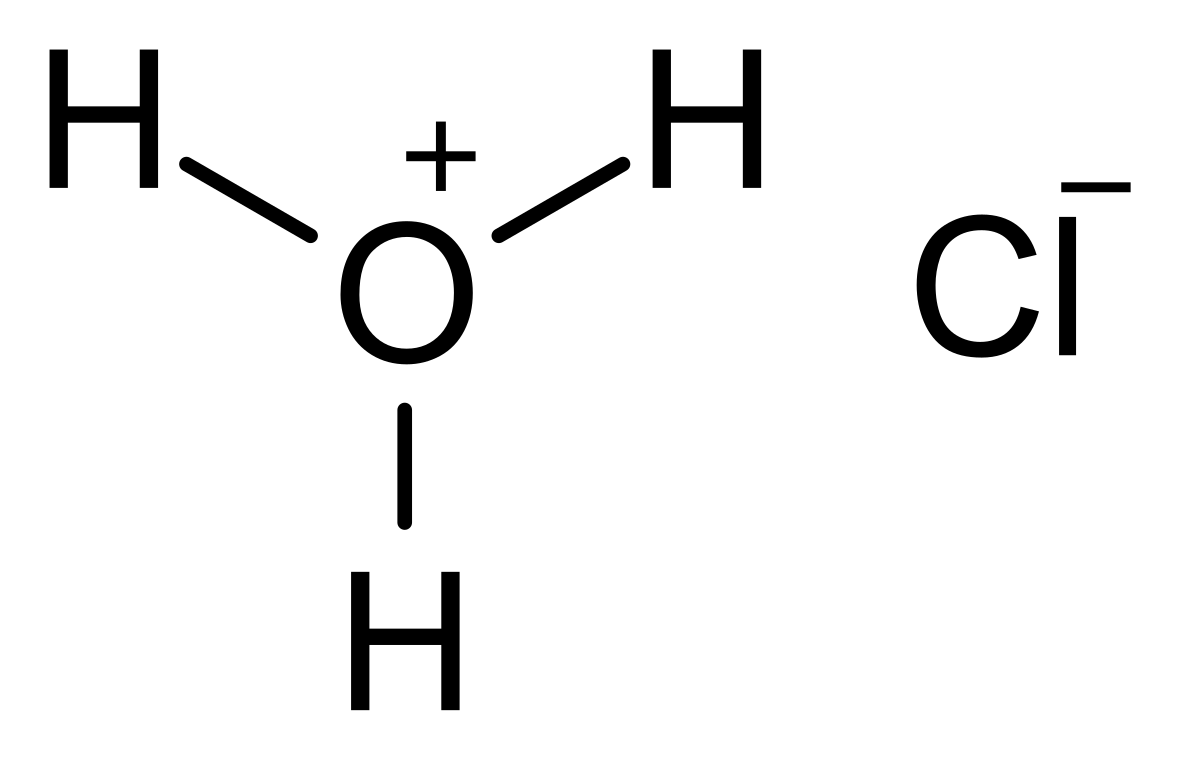
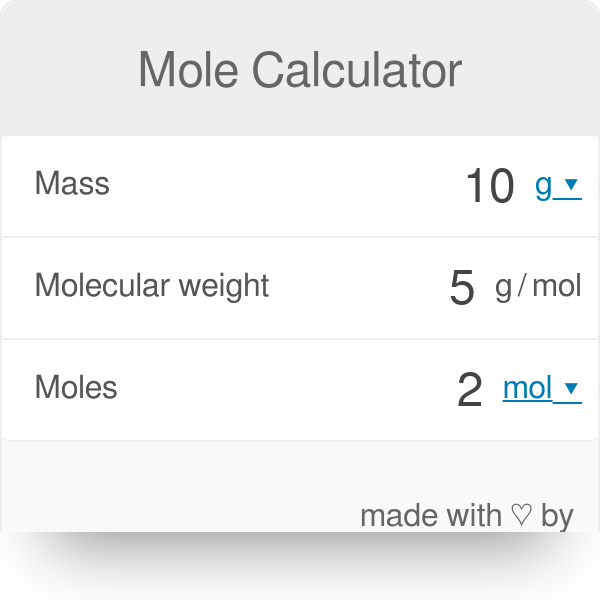
A piece of metallic iron (10 moles) was dissolved in concentrated hydrochloric acid. The reaction formed hydrogen gas and iron chloride. How many grams of HCl were consumed? Don't forget the units. {
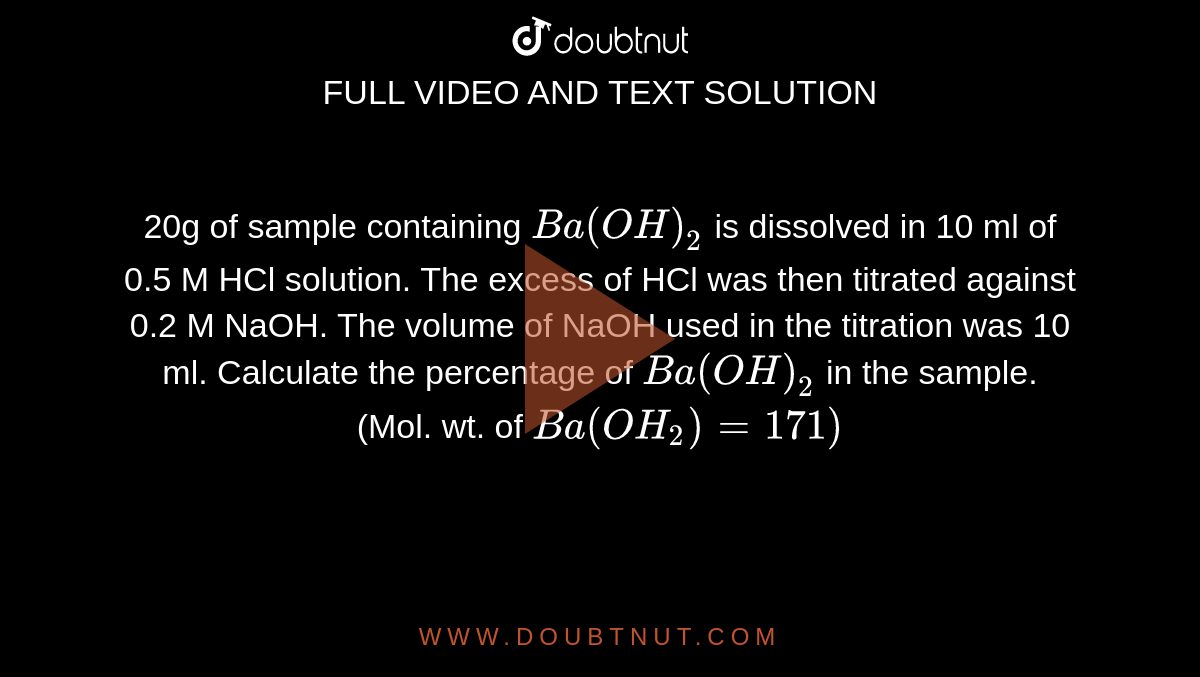
20g of sample containing Ba(OH)(2) is dissolved in 10 ml of 0.5 M HCl solution. The excess of HCl was then titrated against 0.2 M NaOH. The volume of NaOH used in

SOLVED: 1 How many moles of HCl are there in 10 mL of a solution with a concentration of 0.5 molL-I? swered Select one: 5 mole 0.05 mole of 200 Jestion 50 mole 0.5 mole