The dependence of Fe3+ and Fe2+ ions concentration in the solution from... | Download Scientific Diagram

Accelerated Fe3+/Fe2+ cycle using atomic H* on Pd/Al2O3: A novel mechanism for an electrochemical system with particle electrode for iron sludge reduction in the Fe2+/peroxydisulfate oxidation process - ScienceDirect

Fe2+/Fe3+ Ions Chelated with Ultrasmall Polydopamine Nanoparticles Induce Ferroptosis for Cancer Therapy | ACS Biomaterials Science & Engineering

Fe2+/Fe3+ Cycling for Coupling Self‐Powered Hydrogen Evolution and Preparation of Electrode Catalysts - Chen - 2022 - Angewandte Chemie International Edition - Wiley Online Library

Roles of Fe2+, Fe3+, and Cr3+ surface sites in the oxidation of NO on the (Fe,Cr)3O4(1 1 1) surface termination of an α-(Fe,Cr)2O3(0 0 0 1) mixed oxide - ScienceDirect

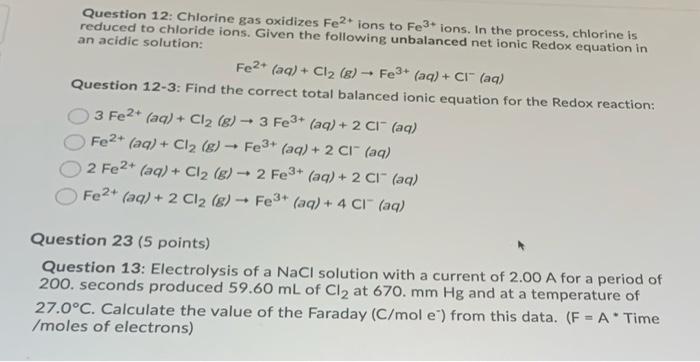
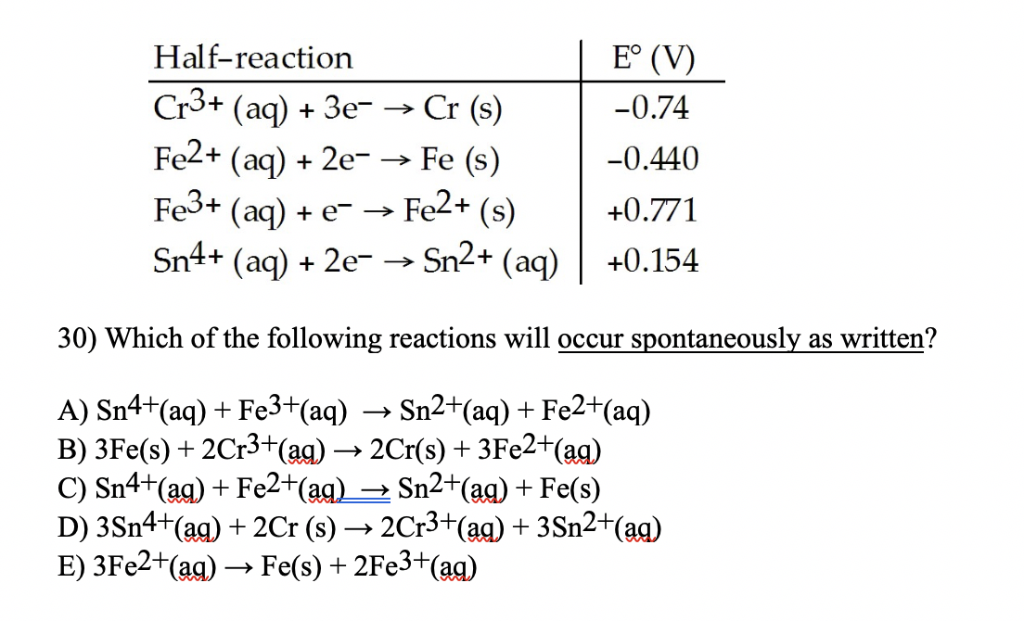
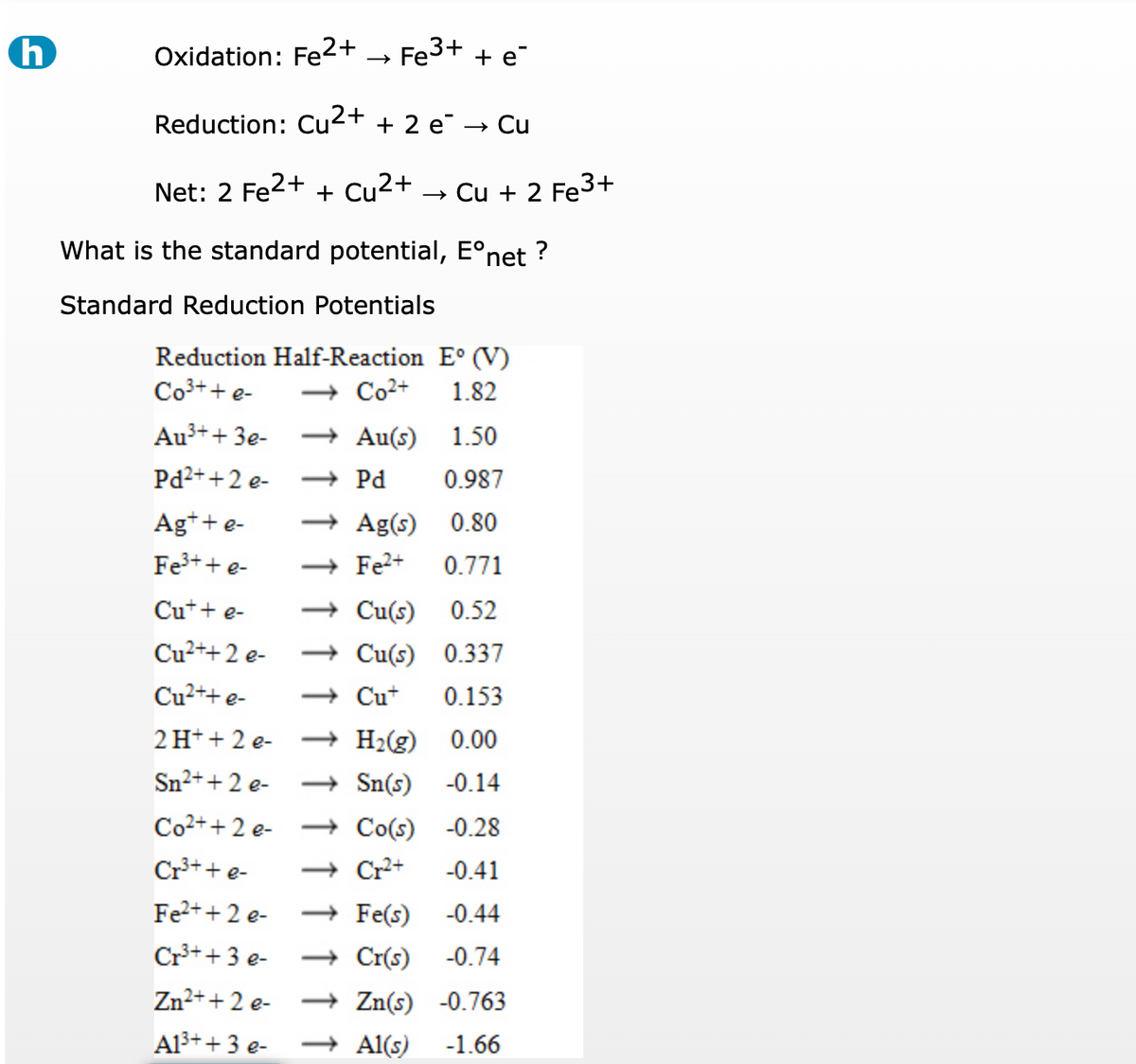
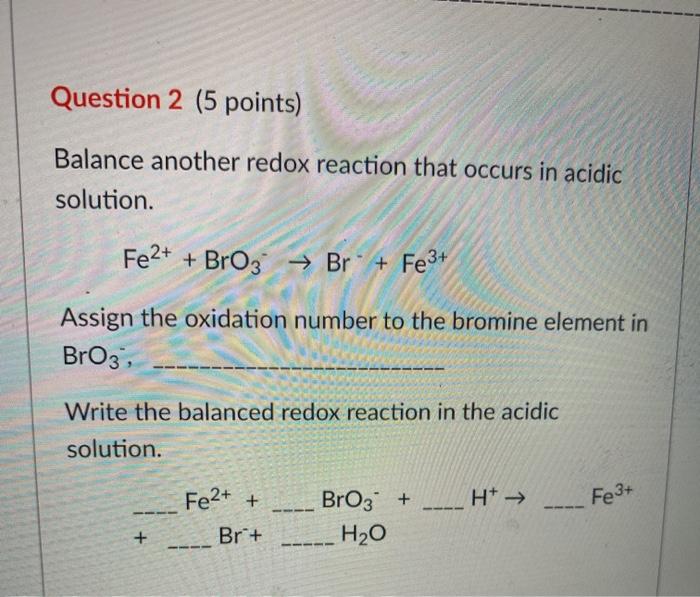
Given standard electrode potentials: Fe^3 + + 3e^-→ Fe;E^0 = - 0.036V Fe^2 + + 2e^-→ Fe;E^0 = - 0.440V The standard electrode potential E^o for Fe^3 + + e^ - → Fe^2 + is:

Aqueous-Phase Differentiation and Speciation of Fe3+ and Fe2+ Using Water-Stable Photoluminescent Lanthanide-Based Metal–Organic Framework | ACS Applied Nano Materials
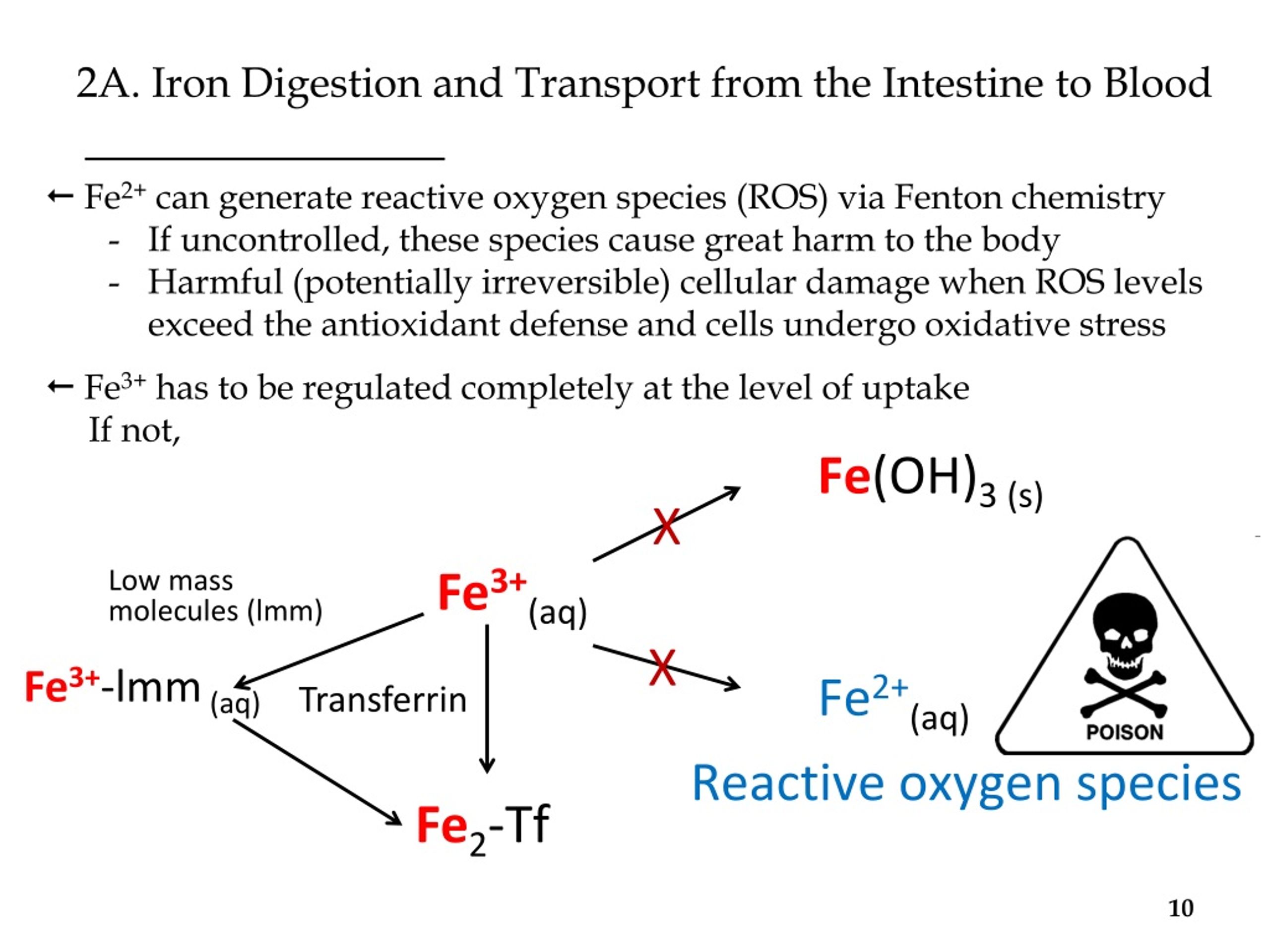
PPT - Iron (Fe 2+ /Fe 3+ ) Transport and Trafficking in Mammals Bertini et al Ch. 5 and 8 PowerPoint Presentation - ID:432903