![Catalytic Production of Hydrogen Peroxide and Water by Oxygen-Tolerant [NiFe]-Hydrogenase during H2 Cycling in the Presence of O2 | Journal of the American Chemical Society Catalytic Production of Hydrogen Peroxide and Water by Oxygen-Tolerant [NiFe]-Hydrogenase during H2 Cycling in the Presence of O2 | Journal of the American Chemical Society](https://pubs.acs.org/cms/10.1021/ja408420d/asset/images/ja408420d.social.jpeg_v03)
Catalytic Production of Hydrogen Peroxide and Water by Oxygen-Tolerant [NiFe]-Hydrogenase during H2 Cycling in the Presence of O2 | Journal of the American Chemical Society

Selenium-Catalyzed Oxidations with Aqueous Hydrogen Peroxide. 2. Baeyer−Villiger Reactions in Homogeneous Solution1 | The Journal of Organic Chemistry

Electrochem | Free Full-Text | Heterostructure of Metal Oxides Integrated on a GCE for Estimation of H2O2 Capacity in Milk and Fruit Juice Samples
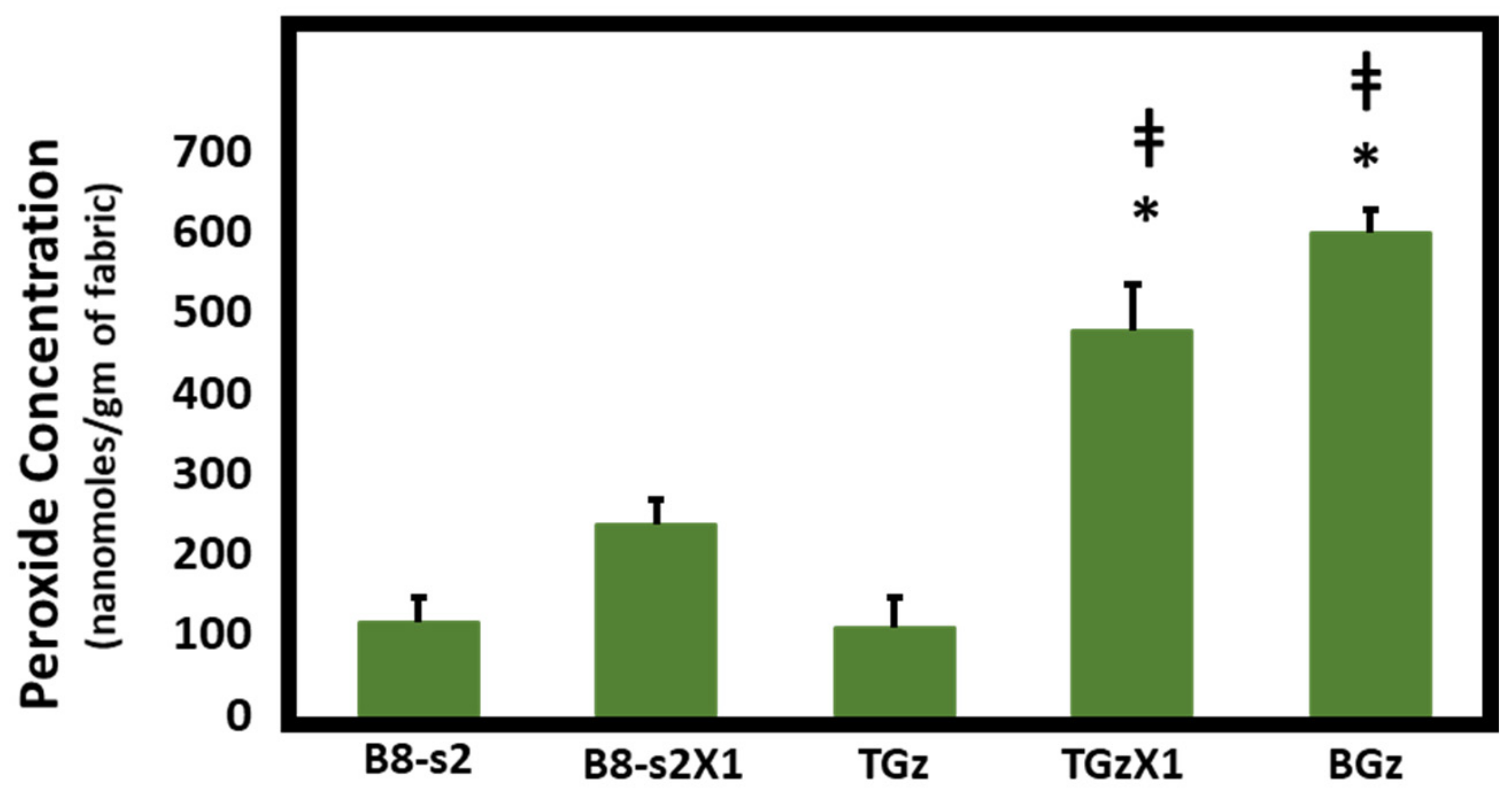
IJMS | Free Full-Text | Ascorbic Acid as an Adjuvant to Unbleached Cotton Promotes Antimicrobial Activity in Spunlace Nonwovens

Highly Efficient Electrochemical Synthesis of Hydrogen Peroxide (H2O2) Enabled by Amino Acid Glycine-Derived Metal-Free Nitrogen-Doped Ordered Mesoporous Carbon | ACS Sustainable Chemistry & Engineering

Raw clay supported ZnO nanoparticles in photodegradation of 2-chlorophenol under direct solar radiations - ScienceDirect

New Multifunctional Skin Care Instrument H2o2 Small Hydrogen Bubble Skin Care Instrument Skin Analysis Test Microdermabrasion - Multi-functional Beauty Devices - AliExpress

DE102006033252B4 - Method and device for measuring humidity and dew point in a hydrogen peroxide-laden environment - Google Patents

Hydrogen Peroxide Decomposition Rate: A Shock Tube Study Using Tunable Laser Absorption of H2O near 2.5 μm | The Journal of Physical Chemistry A
Production of O2 through dismutation of H2O2 during water ice desorption: a key to understanding comet O2 abundances
Schematic view of the initial configuration of the systems H2O H3O + ,... | Download Scientific Diagram





