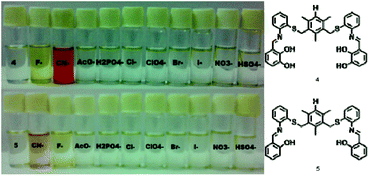
Influence of the composition and concentration of the nutrient solution on plants grown in sand cultures . n> <Z CD n (i I. n 0 c 03 > CI -p COO ?< ?

Calculate Ecell and Δ G for the following at 28^o C Mg(s) + Sn^2 + (0.025M)→ Mg^2 + (0.06M) + Sn(s) E^0cell = 2.23 VIs the reaction spontaneous?

WO2014188488A1 - Electrolytic phosphate salt chemical conversion treatment bath composition and phosphate salt chemical conversion treatment method - Google Patents
![Preparation and characterization of magnetite-dihydrogen phosphate as a novel catalyst in the synthesis of tetrahydrobenzo[b]pyrans - ScienceDirect Preparation and characterization of magnetite-dihydrogen phosphate as a novel catalyst in the synthesis of tetrahydrobenzo[b]pyrans - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0254058417305084-gr1.jpg)
Preparation and characterization of magnetite-dihydrogen phosphate as a novel catalyst in the synthesis of tetrahydrobenzo[b]pyrans - ScienceDirect

Eco-Friendly and Industrially Scalable Synthesis of the Sex Pheromone of Lobesia botrana. Important Progress for the Eco-Protection of Vineyard | Organic Process Research & Development

depicts concentration profiles in the system and defines the various... | Download Scientific Diagram

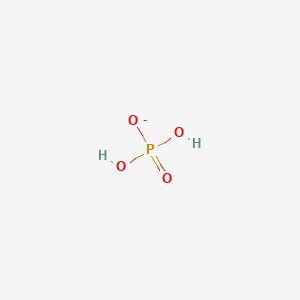
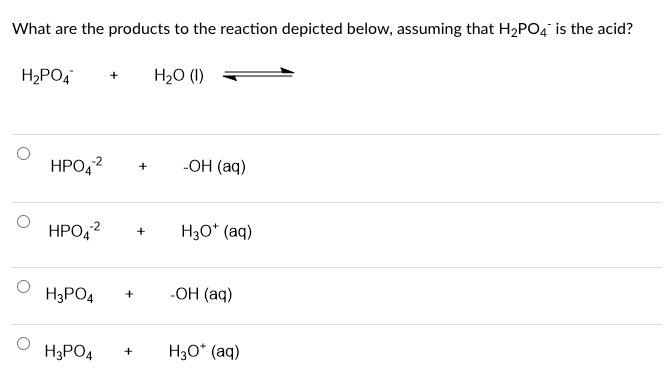
Three reactions involving H2PO4^- are given below:(i) H3PO4 + H2O→H3O^+ + H2PO4^- (ii) H2PO4^- + H2O→HPO4^2 - + H3O^+ (iii) H2PO4^- + OH^-→H3PO4 + O^2 - In which of the above reactions,

Figure 1 from The crystal structure of mercury(I) dihydrogenphosphate, Hg2(H2PO4)2 | Semantic Scholar