Give the correct formula for the compound formed by the combination of the Cu2+ and SO42- ions. | Homework.Study.com

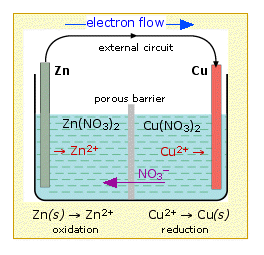
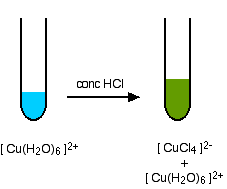
Copper Cu transition metal Chemistry copper(I) Cu+ copper(II) Cu2+ ion complex ions ligand substitution compounds redox chemical reactions principal oxidation states +1 +2 GCE AS A2 IB A level inorganic chemistry revision

Copper Cu transition metal Chemistry copper(I) Cu+ copper(II) Cu2+ ion complex ions ligand substitution compounds redox chemical reactions principal oxidation states +1 +2 GCE AS A2 IB A level inorganic chemistry revision
![In an excess of NH(3(aq.)),Cu^(2+) ion form a deep blue complex ion [Cu (NH(3))(4)]^(2+) having formation constant K(f)=5.6xx10^(11). Calculate the concentration of Cu^(2+) in a solution prepared by adding 5.0xx10^(-3) mole of CuSO(4) In an excess of NH(3(aq.)),Cu^(2+) ion form a deep blue complex ion [Cu (NH(3))(4)]^(2+) having formation constant K(f)=5.6xx10^(11). Calculate the concentration of Cu^(2+) in a solution prepared by adding 5.0xx10^(-3) mole of CuSO(4)](https://d10lpgp6xz60nq.cloudfront.net/ss/web/355853.jpg)
In an excess of NH(3(aq.)),Cu^(2+) ion form a deep blue complex ion [Cu (NH(3))(4)]^(2+) having formation constant K(f)=5.6xx10^(11). Calculate the concentration of Cu^(2+) in a solution prepared by adding 5.0xx10^(-3) mole of CuSO(4)
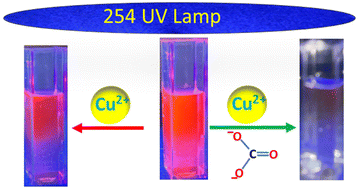
CO32− ion-induced Cu2+ ion determination using DPA capped-LaF3:Eu3+ nanocrystals - Journal of Materials Chemistry C (RSC Publishing)
THE GROUND STATE Cu2+ION AFFINITIES OF GLYCINE, ALANINE AND CYSTEINE IN GAS AND AQUEOUS PHASE: A DFT BASED COMPUTATIONAL STUDY
Predict which of the ions Cu^+, Sc^3+, Mn^2+, Fe^2+ are coloured in aqueous solution? Give reasons. - Sarthaks eConnect | Largest Online Education Community

Colorimetric Detection of Copper(II) Ions Using Schiff‐Base Derivatives - Aydin - 2020 - ChemistrySelect - Wiley Online Library

Why is Cu+ diamagnetic while Cu2+ is paramagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium



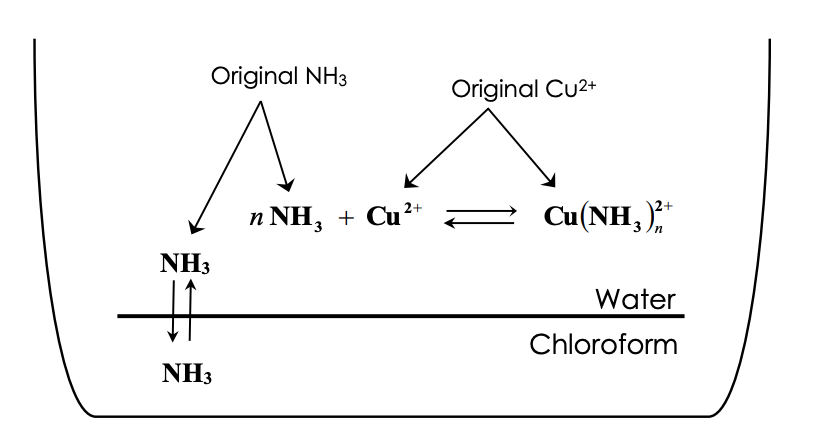


.jpg)