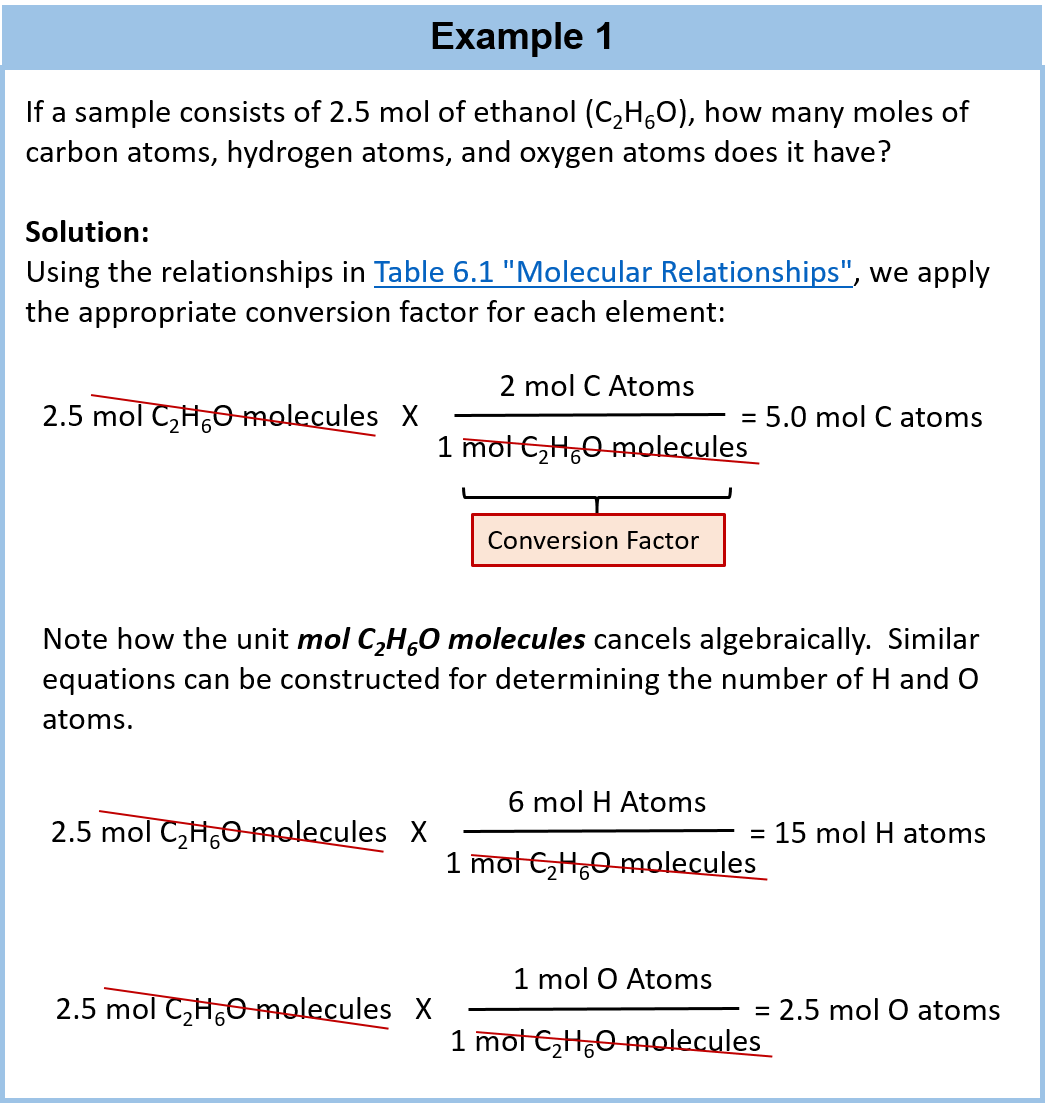
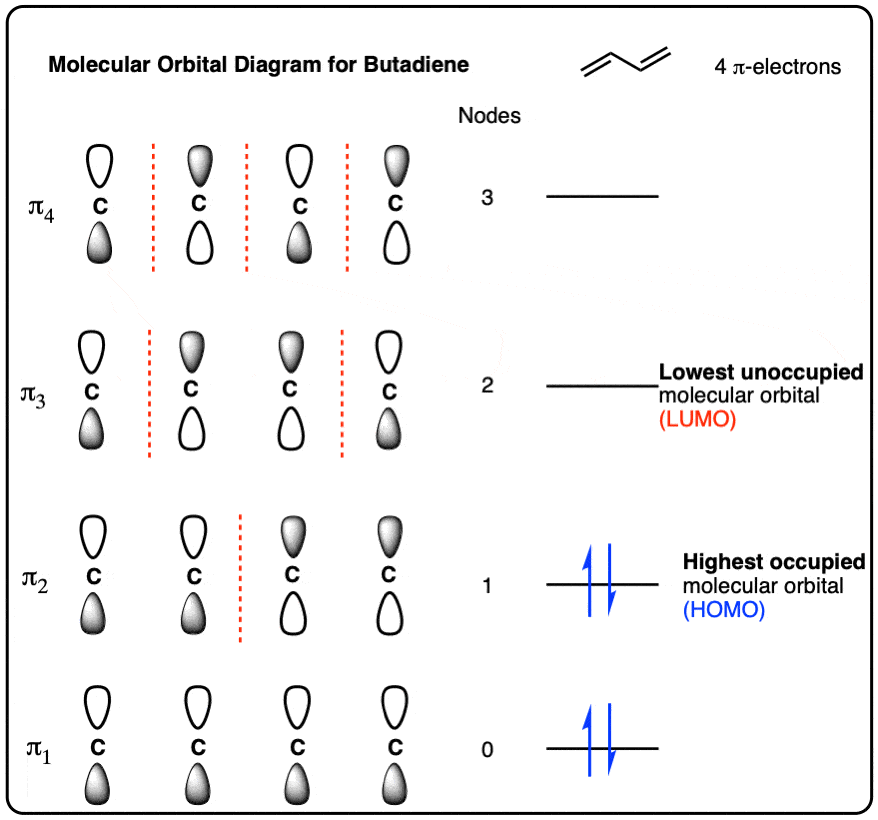
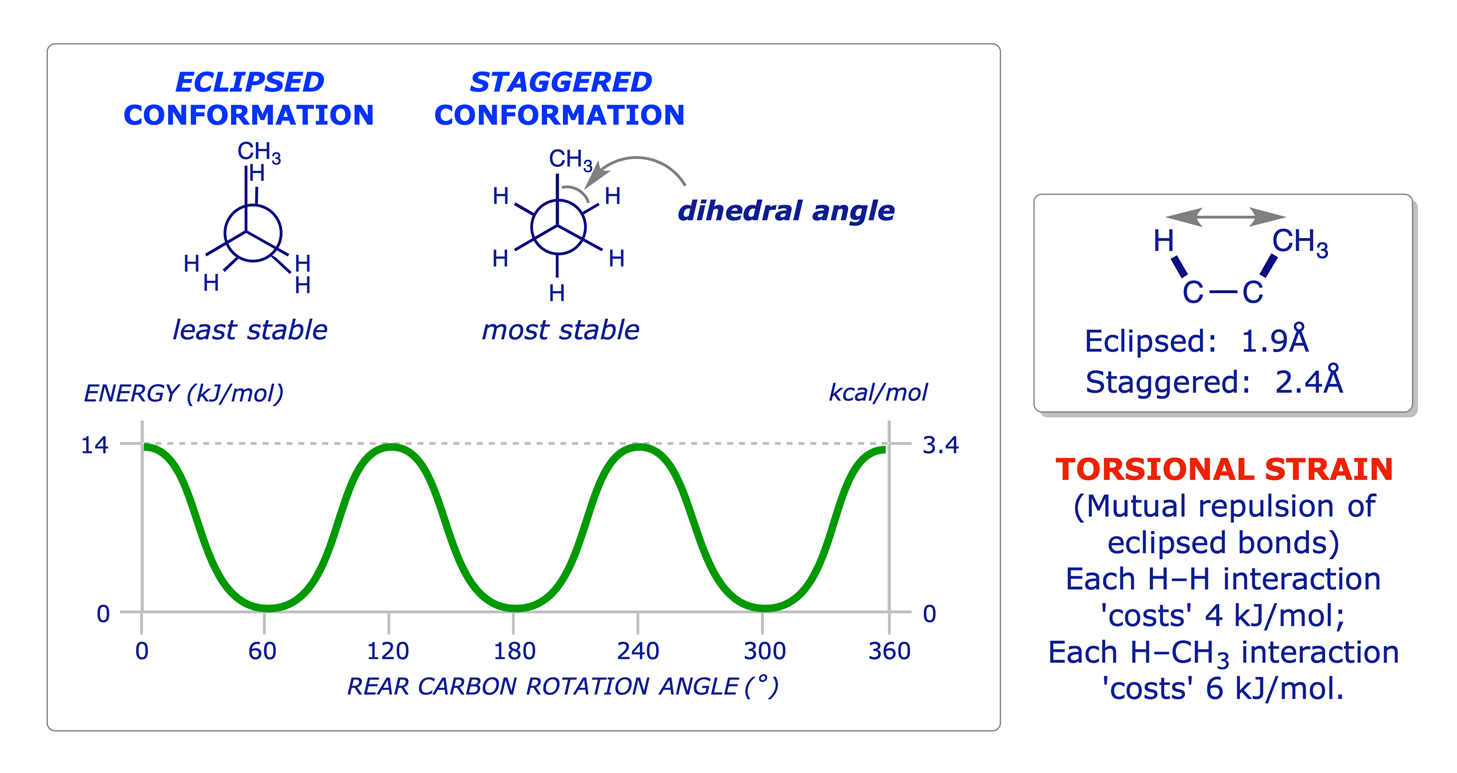
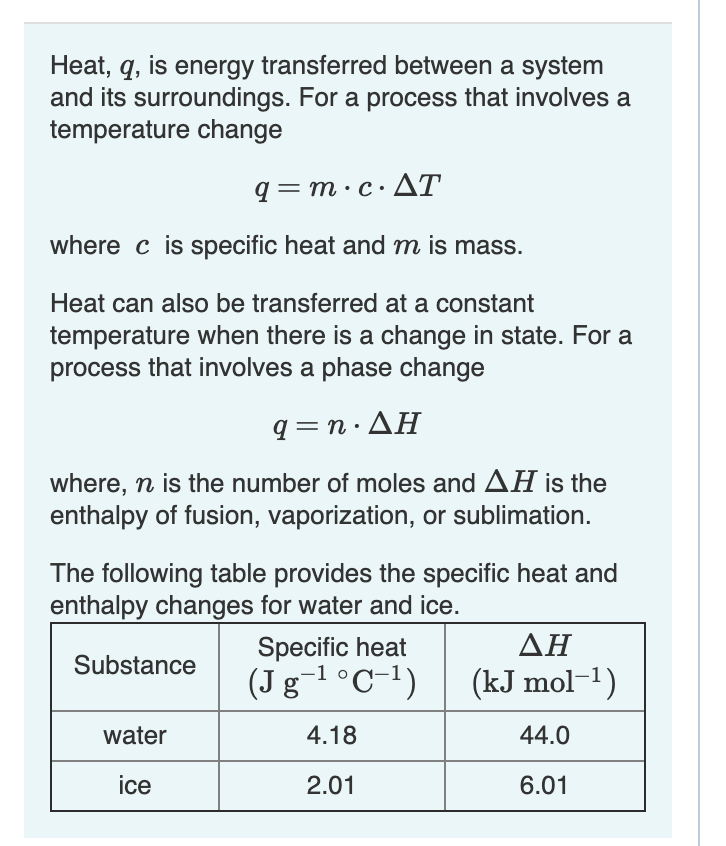
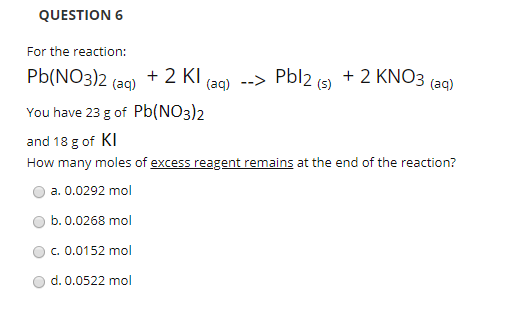SOLVED: A compound of carbon and hydrogen contains 92.3% C and has a molar mass of 78.1 g/mol. Select all that is correct. A. The empirical formula is CH. B. The molecular
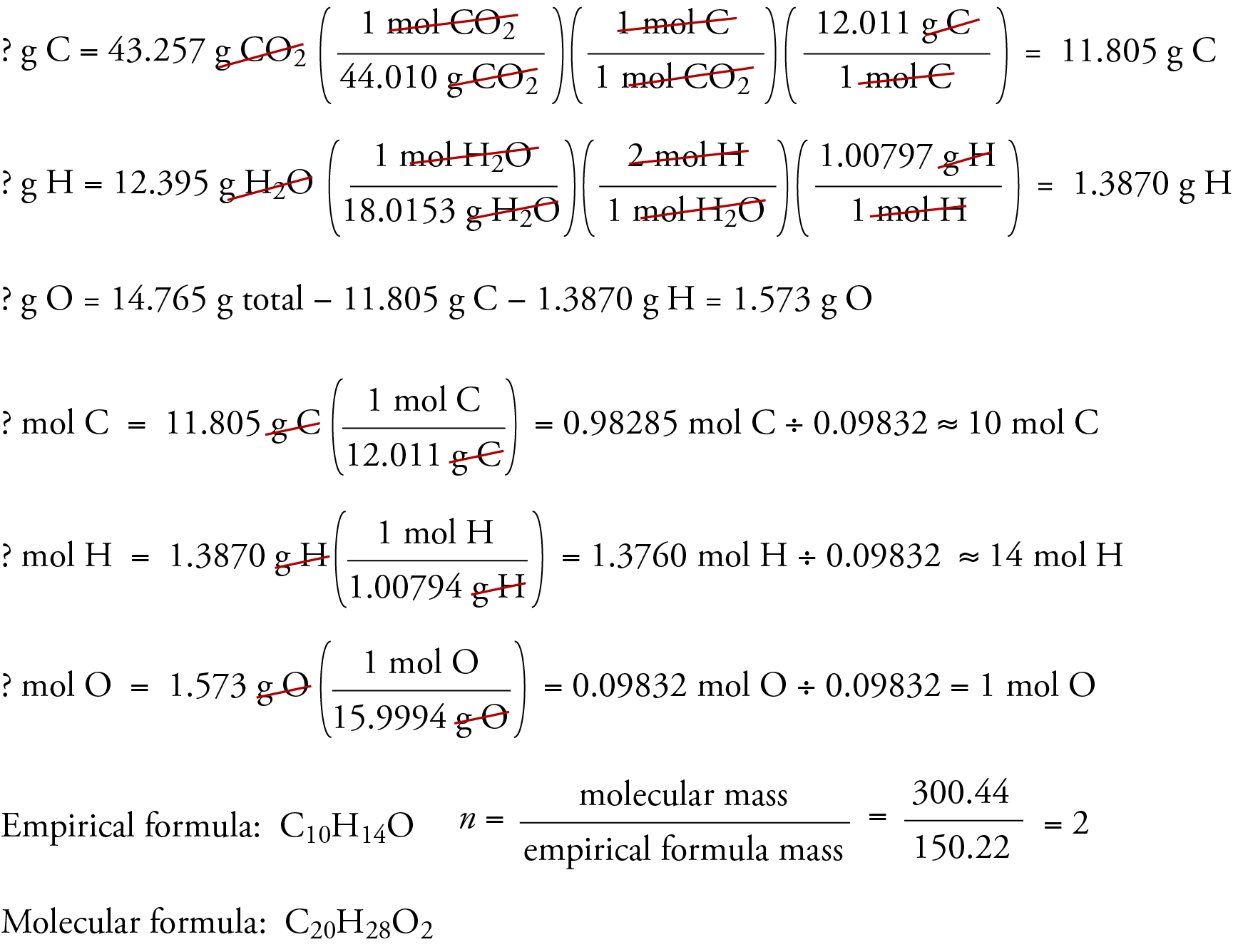
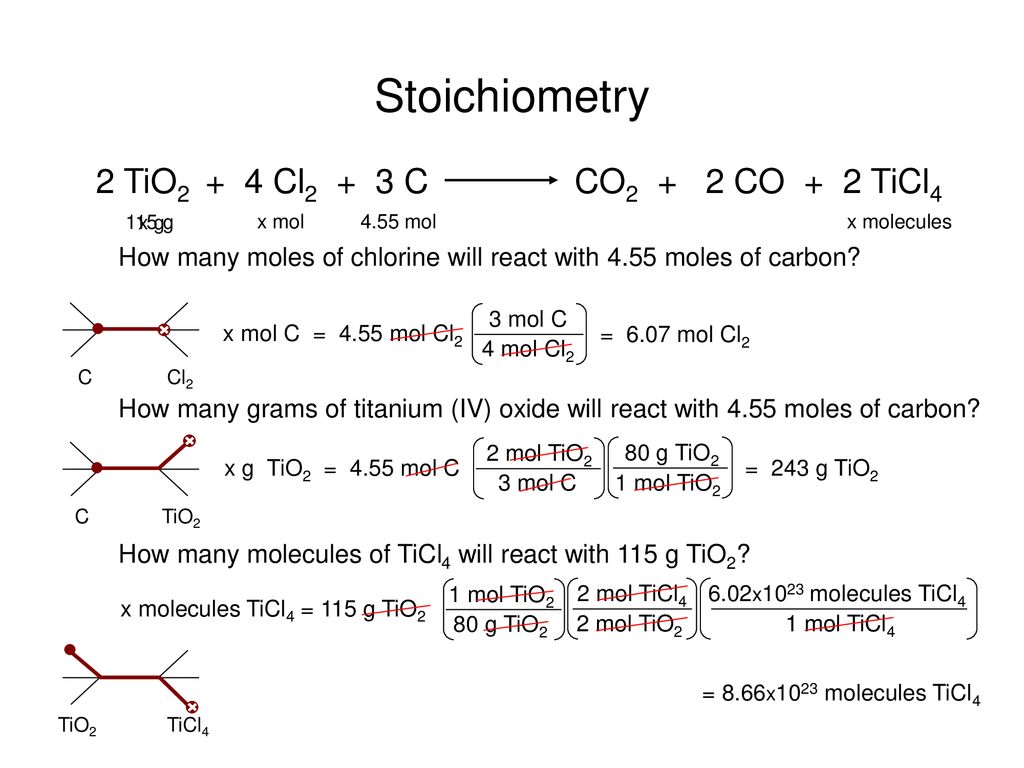
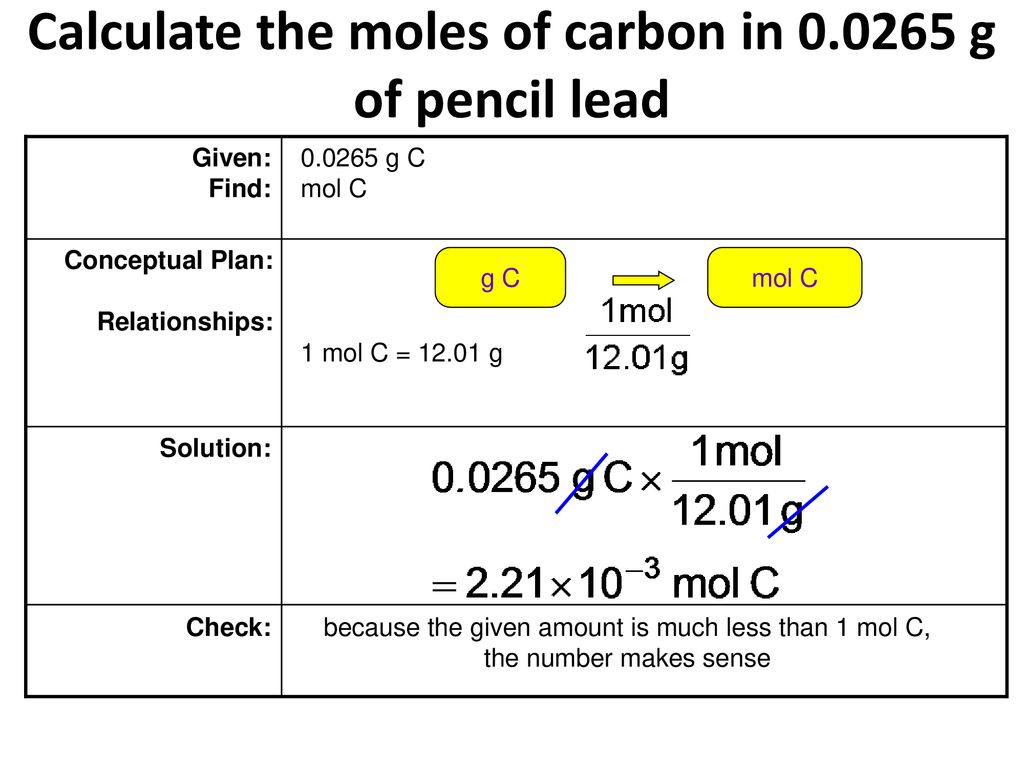
Empirical and molecular formulas for compounds that contain only carbon and hydrogen (C a H b ) or carbon, hydrogen, and oxygen (C a H b O c ) can be determined with a process called combustion analysis. The steps for this procedure are

13.65 | Calculate the number of moles of HI that are at equilibrium with 1.25 mol of H2 and 1.25 mol - YouTube
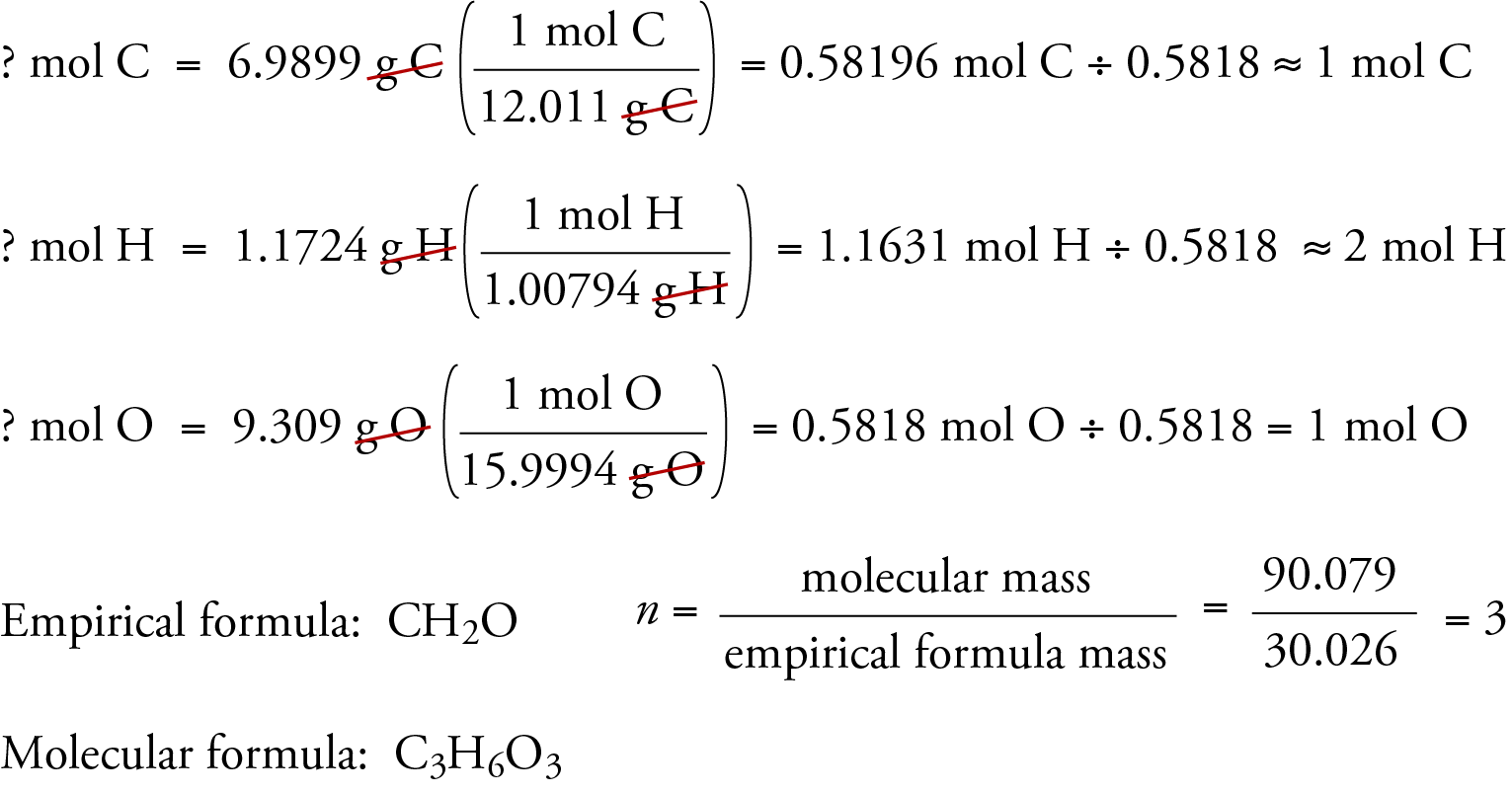
Empirical and molecular formulas for compounds that contain only carbon and hydrogen (C a H b ) or carbon, hydrogen, and oxygen (C a H b O c ) can be determined with a process called combustion analysis. The steps for this procedure are