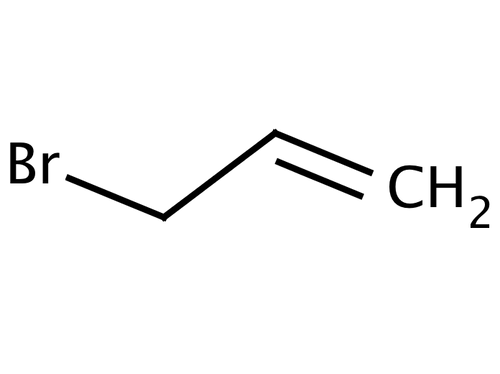
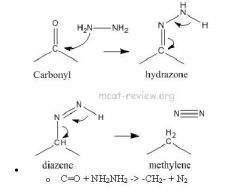
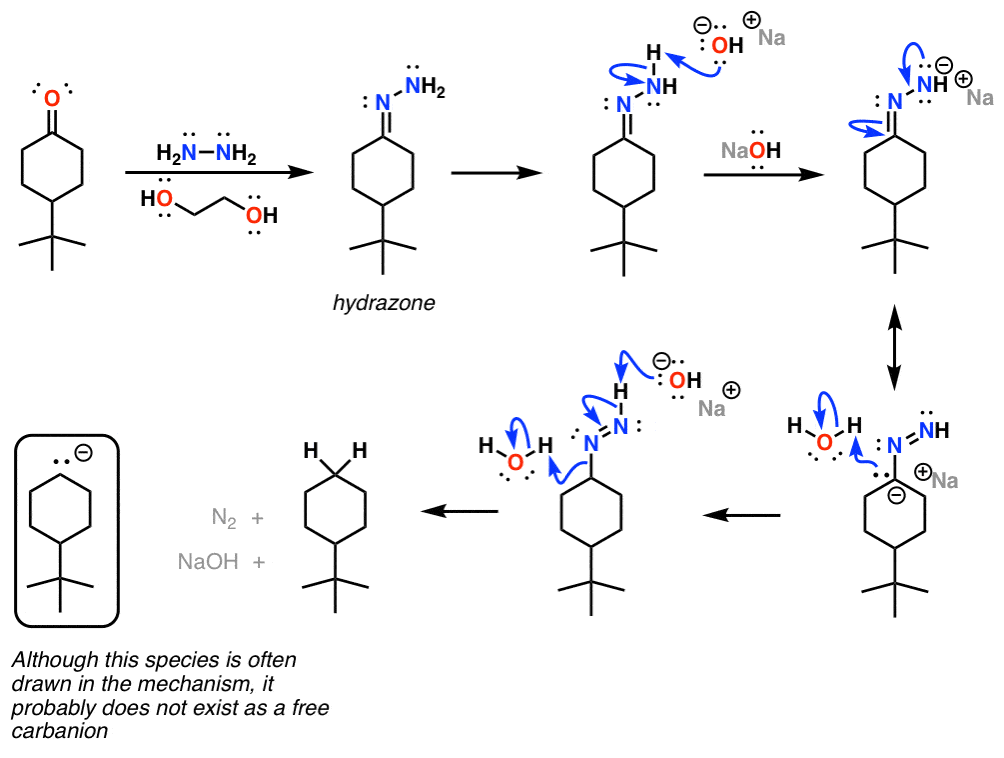
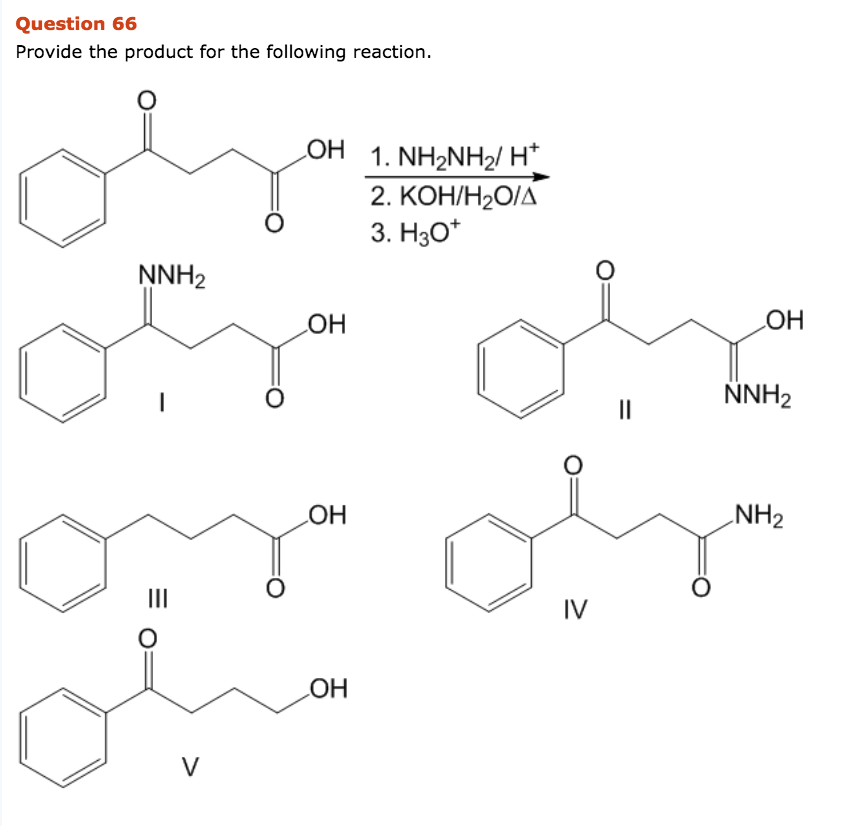
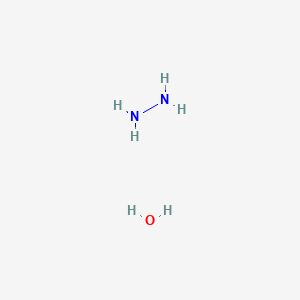
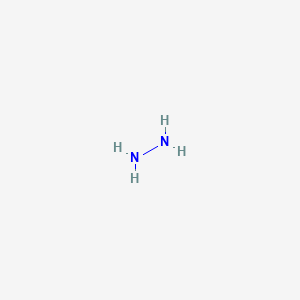
Scheme 3. Reagent and conditions: (i) Isopropyl alcohol, NH2NH2.H2O,... | Download Scientific Diagram

Functionalized 3-hydroxy-3-aminoquinoline-oxindole hybrids as promising dual-function anti-plasmodials - ScienceDirect

Figure 2. Synthesis routes of sulfone derivatives containing 1,3,4-oxadiazole moiety. Reaction conditions and reagents: (a) MeOH, 98%H2SO4, reflux 5h; (b) NH2NH2▫H2O, EtOH, 25°C–reflux, 4h; (c) KOH, CS2, EtOH, 25-46-76°C, 7h; (d) NaOH,

Hydrazine Hydrate 100%,Hydrazine Hydrate Cas ,Hydrazine Hydrate Msds Manufacturers and Suppliers in China
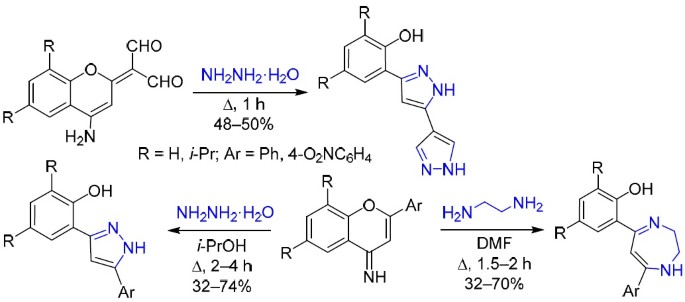
Reactions of 2Н(4Н)-chromenes with dinucleophiles: one-step synthesis of 2-(1H-(bi)pyrazol-3-yl)- and 2-(1,4(5)-(benzo)diazepin-4-yl)phenols | SpringerLink

Reagents and conditions: (a) EtOH, NaHCO3, reflux, 24h; (b) NH2NH2·H2O,... | Download Scientific Diagram
10217-52-4 CAS | HYDRAZINE HYDRATE 24-26% SOLUTION IN WATER | Amines & Amine Salts | Article No. 4080A

Development and Scale-Up of a Continuous Manufacturing Process for a Hydrazine Condensation Reaction | Organic Process Research & Development
CAS 7803-57-8 NH2NH2 · H2O Hydrazine hydrate 80% 化学纯 CP,≥80.0% - CMO,CDMO,Custom Synthesis-Howei Pharm
![Scheme 1, [(i) NH2NH2·H2O, EtOH (ii) PhC(O)CO2Et,...]. - Probe Reports from the NIH Molecular Libraries Program - NCBI Bookshelf Scheme 1, [(i) NH2NH2·H2O, EtOH (ii) PhC(O)CO2Et,...]. - Probe Reports from the NIH Molecular Libraries Program - NCBI Bookshelf](https://www.ncbi.nlm.nih.gov/books/NBK143197/bin/ml228f12.jpg)